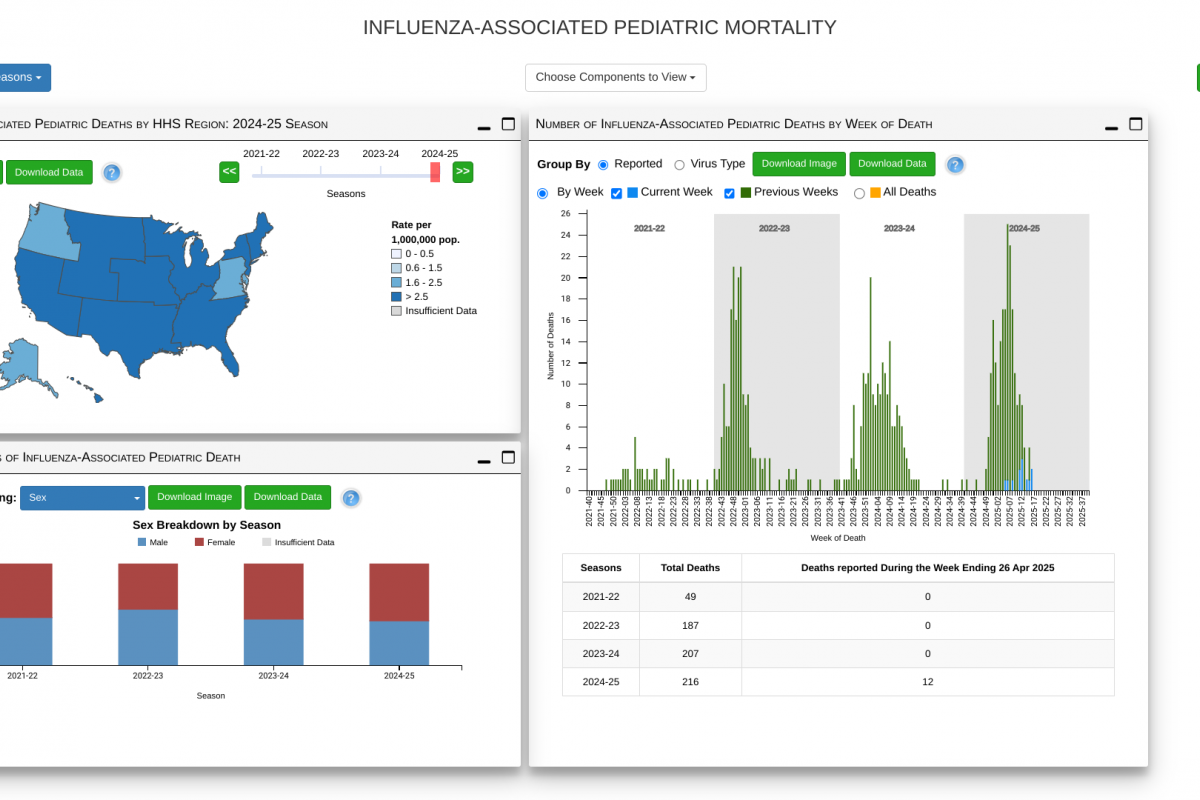
A large-scale, population-based cohort study with a long-term follow-up recently investigated the association between live zoster (shingles) vaccination and the risk of various cardiovascular events.
A study published in the European Heart Journal on May 5, 2025, found a 23% lower risk of cardiovascular events in recipients of the shingles vaccine in the eight years following vaccination, with the most significant reduction observed 2–3 years post-vaccination.
The decrease in cardiovascular disease risk was more pronounced among males, individuals aged less than 60 years, those with unhealthy lifestyle habits, and those from low-income households and rural residents.
"A shingles infection can cause blood vessel damage, inflammation, and clot formation that can lead to heart disease," said study author Dong Keon Yon, PhD, from the Kyung Hee University College of Medicine, Seoul, in a press release.
"By preventing shingles, vaccination may lower these risks."
These findings suggest that live zoster vaccination may be beneficial for reducing the burden of cardiovascular disease in the general population.



.jpg)

.jpg)


_0.png)
.jpg)
.png)