Search API
French Polynesia, an archipelago of over 100 islands and atolls in the South Pacific Ocean, has become a vacationer's paradise. Unfortunately, the mosquito-transmitted Dengue has also found a home in 2025.
As of May 22, 2025, the U.S. Centers for Disease Control and Prevention (CDC) includes French Polynesia in its Level 1 Travel Health Advisory. The CDC says the disease can take up to two weeks to develop, with illness generally lasting less than a week.
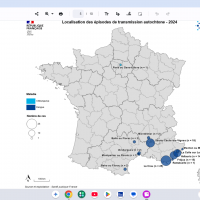
As of April 30, 2025, 1,241 cases of Dengue-like illness were reported this year, five times higher than the 237 cases reported for the same period in 2024.
The majority of recent Dengue cases were from the Windward Islands.
Cumulatively, DENV-1 has become the predominant serotype since the end of 2024.
French Polynesia includes Tahiti, Moorea, Bora-Bora, the Marquesas Islands, and the Austral Islands of Tubuai and Rurutu. In 2025, about 1 million visitors visited French Polynesia, including about 320,000 who visited Tahiti last year.
The CDC says travelers to risk areas should prevent mosquito bites by using an EPA-registered insect repellent and wearing long-sleeved shirts and pants when outdoors.
A second-generation Dengue vaccine is available in some countries in May 2025, but not in the United States.
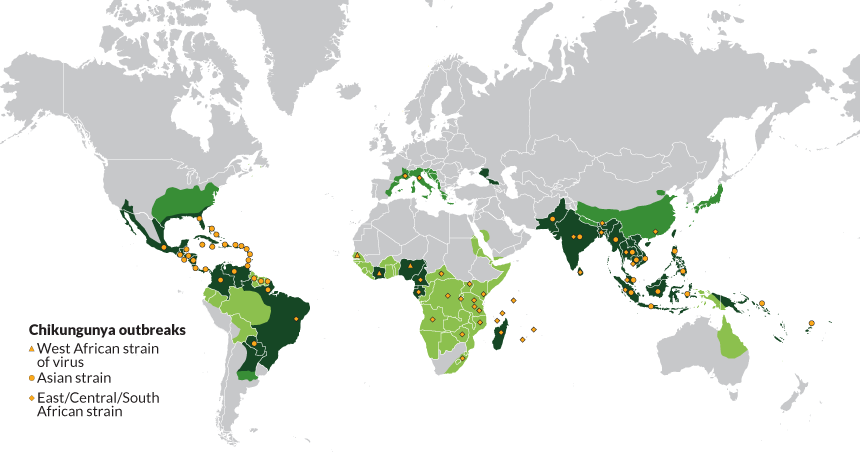
Certain vector-borne diseases, such as Chikungunya, Dengue, and Zika, are expanding public health challenges in Southeast Asia, including the Kingdom of Thailand. There has been evidence of chikungunya virus transmission in Thailand within the last five years.
These diseases have become a health risk to Thailand's 35 million visitors annually.
A research article published in the Journal of Infectious Diseases on May 21, 2025, assessed the role of sylvatic reservoirs in arboviral circulation by examining serological evidence of exposure to DENV, ZIKV, and CHIKV among humans and macaques living close in endemic regions.
The overall seropositivity rates across arboviruses among human populations are higher than those of macaques (38.5-74.4% versus 0-8.0%, respectively).
Globally, a study published in April 2025 estimates that 5.66 billion (95% confidence interval 5.64-5.68) people currently live in areas suitable for Dengue, Chikungunya, and Zika.
The U.S. CDC says international travelers should speak with a vaccine expert before visiting Thailand in 2025 regarding routine and travel vaccine options, such as Valneva SE's IXCHIQ® Chikungunya vaccine. These vaccines are offered at travel clinics and pharmacies and should be administered weeks before departing abroad in 2025.
After decades of progress against cholera, cases are increasing even in countries in Southeast Asia that had not seen the disease in years.
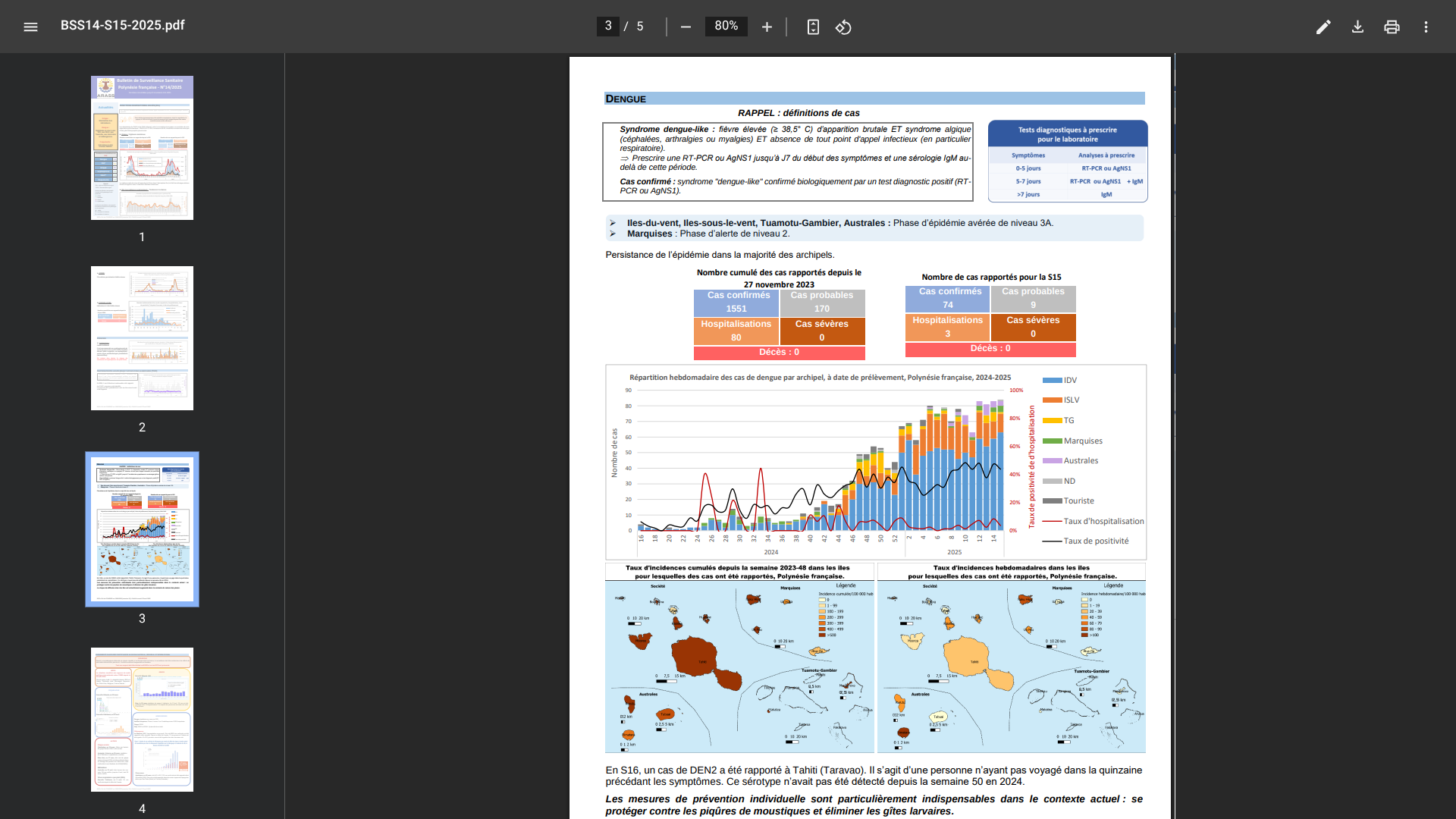
According to the World Health Organization (WHO) external situation report #26, published on May 22, 2025, Bangladesh, India, Myanmar, Nepal, and Thailand reported additional cholera cases in April 2025.
From January to late April 2025, 1,868 cholera / AWD cases were reported across five countries in the Southeast Asia Region. The highest number of cases was reported from India (11,730), Myanmar (8,869), Bangladesh (655), Nepal (445), and Thailand (16).
Furthermore, a total of 58 cholera-related deaths were reported in India.
The WHO says cholera is a vaccine-preventable disease.
However, as of May 19, 2025, the global Oral Cholera Vaccine (OCV) stockpile stands at 3.6 million doses, below the minimum emergency threshold of five million.
OCVs are approved and available at travel clinics and pharmacies in the United States.
With the mosquito-transmitted Chikungunya virus infecting over 144,000 residents and international travelers throughout the Region of the Americas in 2025, access to an approved vaccine may soon expand.
According to Valneva SE's website, on May 23, 2025, Valneva wrote, 'By 2026, we want to enable access to Valneva's single-shot chikungunya vaccine IXCHIQ® in India and Brazil by enabling local manufacturing and access through technology transfers.'
'In Asia, we signed an exclusive license agreement with the Serum Institute of India, complementing our existing agreement with Instituto Butantan in the Americas.'
IXCHIQ was the first vaccine approved in 2023 to address chikungunya virus infections in adults at increased risk of exposure to the mosquito-transmitted disease. This vaccine is currently available in the United States at clinics and pharmacies.
The U.S. CDC has issued travel health notices for outbreaks in Mauritius, Mayotte, Réunion, Somalia, and Sri Lanka to warn international travelers of the Chikungunya health risk. Vaccination is recommended for travelers visiting an area with an outbreak.
Thanks to the world's first vaccination program against a leading sexually transmitted infection (STI), thousands of gonorrhoea cases in the United Kingdom could be prevented over the next decade.
The UK's NHS and local government announced on May 21, 2025, that they are launching a vaccine program to prevent the recent increase in gonorrhoea cases. Those who receive the meningococcal B disease vaccine 4CMenB (Bexsero®) could be protected from gonorrhoea by up to 40%.
In 2023, there were 85,000 gonorrhoea diagnoses in England, 300% higher than in 2012.
Eligible patients will be offered the vaccine through local authority-commissioned sexual health services from early August 2025.
Eligible people will also be offered mpox (JYNNEOS), hepatitis A and B, and human papillomavirus vaccinations when attending their appointment for the gonorrhoea vaccine.
James Woolgar, Chair of English HIV and Sexual Health Commissioners’ Group, commented in a press release, “Introducing the world’s first gonorrhoea vaccine programme into England’s sexual health services is a major milestone for public health."
In the United States, Bexsero is FDA-approved, recommended by the CDC for certain people, and available at most clinics and pharmacies.

The U.S. FDA published the Briefing Document for the Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting on May 22, 2025. VRBPAC has met multiple times since 2022 to discuss and make recommendations on selecting the strain compositions for COVID-19 vaccines.
Today's meeting agenda focused on selecting the 2025-2026 Formula for COVID-19 Vaccines.
As noted in the Document, while current circulating SARS-CoV-2 variants have derived from the JN.1 variant that appeared in late 2023, the JN.1 virus lineage continues to evolve.
The LP.8.1 subvariant has now become the predominant circulating strain, but other virus subvariants including LF.7 and XFG have also been increasingly detected in the U.S. in the recent weeks.
Because of the continuing antigenic drift between JN.1 and KP.2, which were used in the 2024-2025 vaccine, and the currently circulating subvariants in the Asia Pacific countries, a review and discussion regarding the need for a strain composition update for COVID-19 vaccines is warranted.
In an article published in the New England Journal of Medicine on May 20, 2025, FDA Commissioner Dr. Martin Makary and Dr. Vinay Prasad, director of the FDA's Center for Biologics Evaluation and Research, stated that these changes align the U.S. with the practices of many other countries.
'The FDA's new COVID-19 philosophy represents a balance of regulatory flexibility and a commitment to gold-standard science. The FDA will approve vaccines for high-risk persons and, at the same time, demand robust, gold-standard data on persons at low risk.'
'These clinical trials will inform future directions for the FDA, but more importantly, they will provide information that health care providers and the American people desperately crave.'
The complete FDA Briefing Document is available at this link.
-3.jpg)
The New Jersey Department of Health (NJDOH) recently informed residents about a potential exposure linked to a newly identified case of measles in a non-New Jersey resident who attended the Shakira concert at the Hackensack Meadowlands while infectious.
On May 20, 2025, the NJDOH recommended that anyone who visited the MetLife Stadium from May 15, 2025, 7:30 PM through May 16, 2025, 1:00 AM and suspects an exposure or illness call a healthcare provider before going to any medical office.
NJDOH says Individuals – especially parents, guardians, health care providers, and caregivers – are urged to be aware of the symptoms of this highly contagious virus and to ensure they are up to date with the measles, mumps, and rubella (MMR) shots.
As of May 22, 2025, MMR vaccines are offered at most clinics and pharmacies in NJ.

Over the past few weeks, COVID-19 cases have surged across Asia, with Hong Kong and Singapore reporting spikes.
The current COVID-19 infection wave is unfolding as the annual respiratory season usually subsides. It has been linked to the JN.1 variant, which was first identified in 2023.
However, as of May 21, 2025, health officials in India believe they have the situation under control.
India's Ministry of Health and Family Welfare (MoHFW) recently stated that 'the current situation in India is “under control,” with only 257 active cases reported.'
This latest resurgence of COVID-19 is a real-world reminder that the SARS-CoV-2 coronavirus remains a public health threat worldwide.
When visiting India in May 2025, the U.S. CDC writes, ' All eligible travelers should be up to date with their COVID-19 vaccines.'
Additionally, the CDC suggests routine vaccinations and says there has been evidence of chikungunya virus transmission in India within the last 5 years. Cholera is also presumed to be present in India, and Japanese encephalitis vaccination is recommended when visiting endemic areas.
These travel vaccines are offered in the U.S. at clinics and pharmacies.
-2.png)
The Texas Department of State Health Services (DSHS) today reported the measles outbreak now totals 722 cases, with 92 people hospitalized, and two related fatalities.
As of May 20, 2025, DSHS reported 15 additional measles cases, led by Harris County, including Houston.
Similar to the outbreaks in Ontario, Canada, which have resulted in 1,846 measles cases in 2025, the vaccination status of the individuals in West Texas remains unknown.
However, Texas also disclosed positive data regarding school-age vaccinations.
Texas requires public school districts and accredited private schools to submit a report of their students’ immunization status every year.
In seventh grade, immunization coverage was above 95% for all vaccines except Tdap and meningococcal MCWY vaccines.
In Texas, people can get vaccinated by going to their healthcare provider or a pharmacy. Vaccines are also available through the Texas Vaccines for Children and Adult Safety Net Providers.