U.S. Changes COVID-19 Vaccination Policy

The U.S. Food and Drug Administration (FDA) announced updates to its COVID-19 vaccination recommendations today, as the U.S. population has grown tired of the current policy.
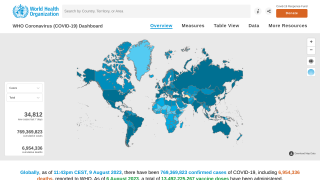
According to the Centers for Disease Control and Prevention, fewer than 25% of Americans received boosters yearly over the past two seasons.
Even healthcare workers are hesitant about vaccination, with less than one-third participating in the 2023–2024 fall booster program.
In an article published in the New England Journal of Medicine on May 20, 2025, FDA Commissioner Dr. Martin Makary and Dr. Vinay Prasad, director of the FDA's Center for Biologics Evaluation and Research, stated that these changes align the United States with the practices of many other countries.
'While all other high-income nations confine vaccine recommendations to older adults (typically those older than 65 years of age), or those at high risk for severe COVID-19, the U.S. (government) has adopted a one-size-fits-all regulatory framework and has granted broad marketing authorization to all Americans over the age of six months.'
'The U.S. policy has sometimes been justified by arguing that the American people are not sophisticated enough to understand age- and risk-based recommendations.
'We reject this view,' wrote these FDA leaders.
'The FDA's new COVID-19 philosophy represents a balance of regulatory flexibility and a commitment to gold-standard science. The FDA will approve vaccines for high-risk persons and, at the same time, demand robust, gold-standard data on persons at low risk.'
'These clinical trials will inform future directions for the FDA, but more importantly, they will provide information that health care providers and the American people desperately crave.'
This entire, unedited article is posted at this link.
Our Trust Standards: Medical Advisory Committee








.jpg)

.jpg)



.jpg)