New Vaccine Recommendations Published

In the United States, the Advisory Committee on Immunization Practices (ACIP) develops recommendations on how to use vaccines to control disease. Once adopted, these ACIP recommendations become the official policy of the U.S. Centers for Disease Control and Prevention (CDC).
As of May 15, 2025, the CDC published a user-friendly listing of the last five ACIP meeting recommendations.
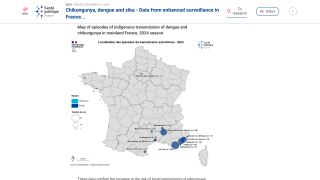
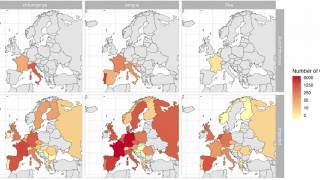
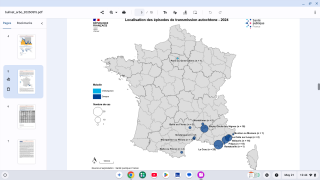
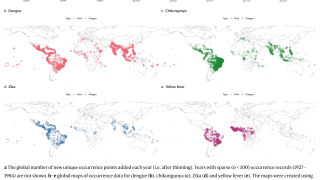
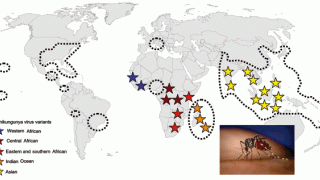
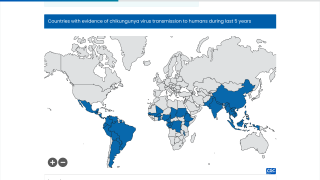
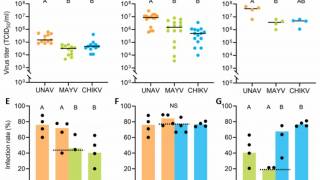
Recently, U.S. Department of Health and Human Services Secretary Robert F. Kennedy Jr. signed off on three vaccine recommendations passed last month by the ACIP. These actions include approvals for Chikungunya vaccines.
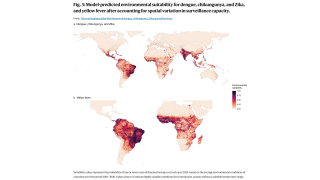
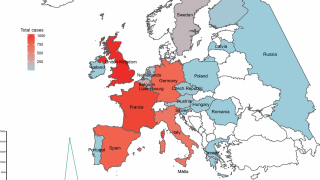
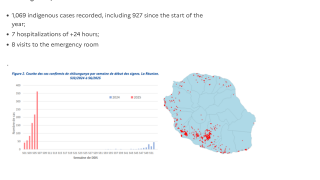
These approvals are essential to reduce the ongoing Chikungunya outbreaks in the Indian Ocean and the Region of the Americas.
Other ACIP recommendations related to a new meningococcal vaccine and RSV vaccines are pending as of May 19, 2025.
Additionally, to promote broader awareness, these recommendations are published in the CDC’s Morbidity and Mortality Weekly Report.
To learn about historical ACIP vaccine recommendations for various diseases, visit this CDC webpage.
Our Trust Standards: Medical Advisory Committee


-2.png)
-2.jpg)



.jpg)