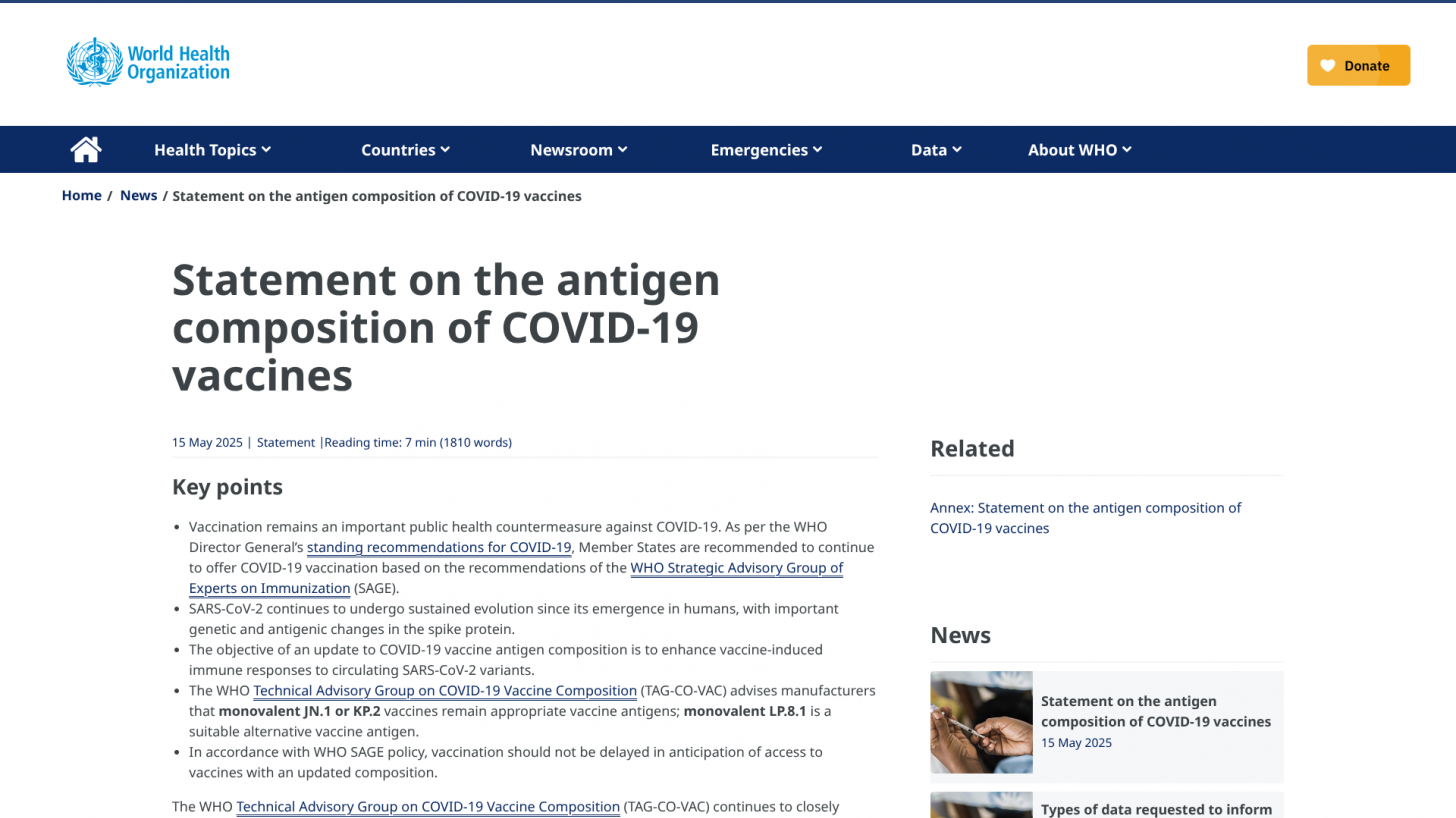
The WHO Clarifies Antigen Composition of COVID-19 Vaccines
The World Health Organization (WHO) Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) today confirmed it continues to closely monitor the genetic and antigenic evolution of SARS-CoV-2 variants, immune responses to SARS-CoV-2 infection and COVID-19 vaccination, and the performance of COVID-19 vaccines against circulating variants.
As of May 2025, the currently circulating SARS-CoV-2 variants are derived from JN.1.
Multiple manufacturers (using mRNA, recombinant protein-based, and adenovirus-vectored platforms) have updated their COVID-19 vaccines, such as Novavax Inc. Novavax's recombinant protein-based vaccine is the only non-mRNA COVID-19 vaccine available in the United States.
On May 15, 2025, the TAG-CO-VAC advised manufacturers that monovalent JN.1 or KP.2 vaccines remain appropriate vaccine antigens; monovalent LP.8.1 is a suitable alternative vaccine antigen.
The WHO says, 'The objective of an update to COVID-19 vaccine antigen composition is to enhance vaccine-induced immune responses to circulating SARS-CoV-2 variants.'
'Overall, the currently approved monovalent JN.1 or KP.2 vaccines continue to elicit broadly cross-reactive immune responses to circulating JN.1-derived variants.'
As previously stated, the TAG-CO-VAC continues to encourage the further development of vaccines that may improve protection against infection and reduce transmission of SARS-CoV-2. Previous statements from the TAG-CO-VAC can be found on the WHO website.
Our Trust Standards: Medical Advisory Committee









.jpg)

.jpg)



.jpg)