66% of Infants Protected Against RSV
As the current respiratory syncytial virus (RSV) season ends, most infants are protected against this severe disease.
According to the U.S. CDC's MMWR ( 74(16);273–281) published on May 8, 2025, in the first RSV season with widespread availability of maternal vaccine and long-acting monoclonal antibody, RSV-associated hospitalization rates among infants were lower than in prepandemic seasons.
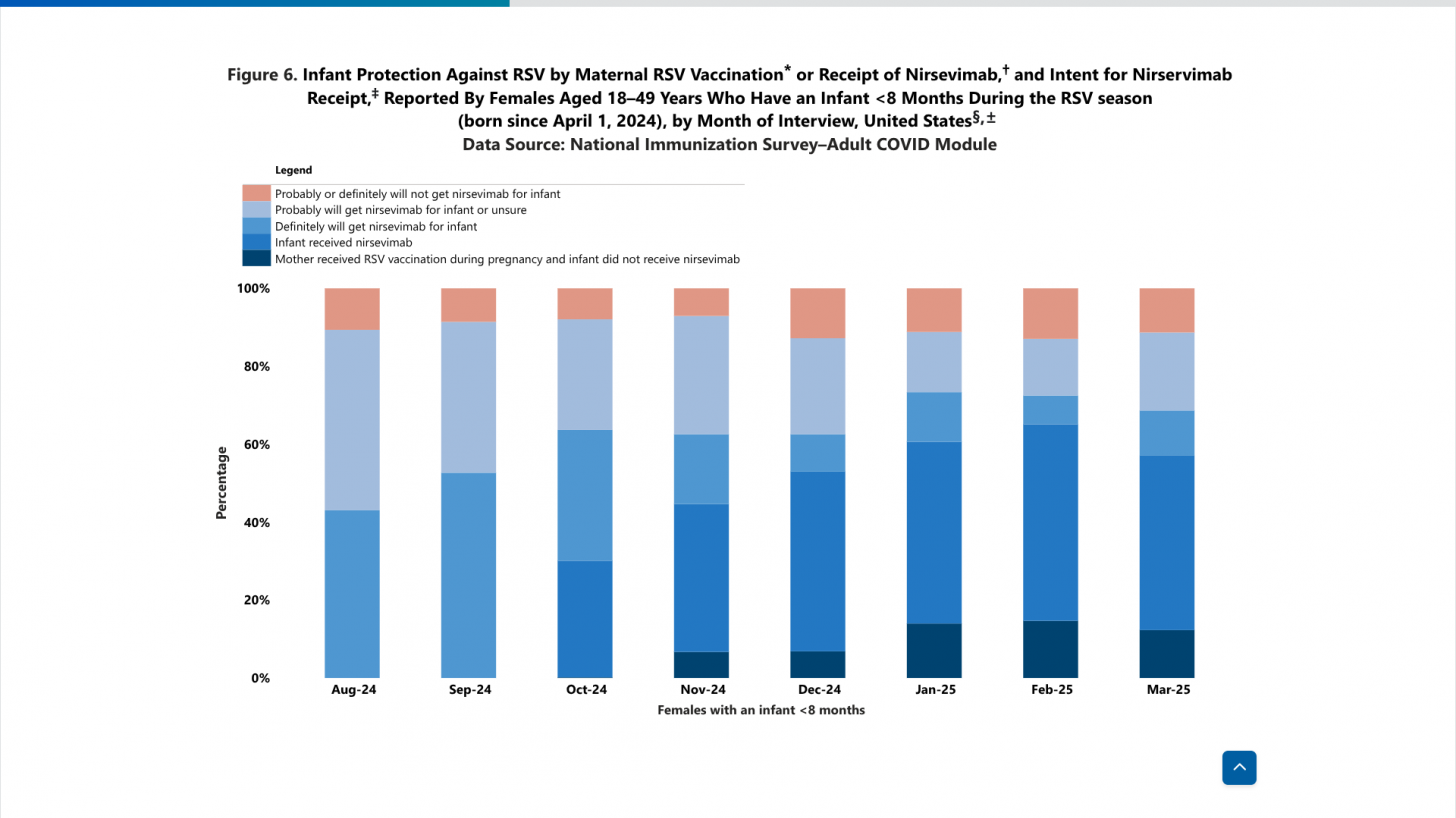
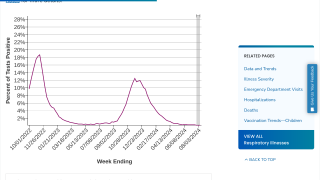
National immunization survey data indicate that the estimated proportion of U.S. infants aged 0–7 months protected by maternal vaccination or nirsevimab increased during the 2024–25 RSV season, from 30% in October 2024 to 66% in February 2025.
Maternal RSV vaccines and nirsevimab (Beyfortus) for infants became widely available for prevention of severe RSV disease among infants and young children during the 2024–25 RSV season.
To evaluate the association between the availability of these products and infant and child RSV-associated hospitalization rates, the rates among children aged <5 years were compared for the 2024–25 and 2018–20 RSV seasons.
Among infants aged 0–7 months (eligible for protection with maternal vaccination or nirsevimab), 2024–25 RSV-associated hospitalization rates were lower compared with 2018–20 pooled rates (estimated relative rate reductions of 43% [RSV-NET: 95% CI = 40%–46%] and 28% [NVSN: 95% CI = 18%–36%]).
The largest estimated rate reduction was observed among infants aged 0–2 months (RSV-NET: 52%, 95% CI = 49%–56%; NVSN: 45%, 95% CI = 32%–57%) and during peak hospitalization periods (December–February).
The CDC wrote that these findings highlight the importance of implementing the CDC's recommendations to protect infants as early in the RSV season as possible, before peak transmission, and for infants born during the RSV season, within the first week of life, ideally during the birth hospitalization.
Data: Monthly estimates of infant protection against RSV by maternal RSV vaccination or receipt of nirsevimab, as well as intent for nirsevimab receipt, reported by adult females aged 18-49 years, are from the National Immunization Survey─Adult COVID Module (NIS─ACM). Overall, 18,389 RSV-associated hospitalizations (15,405 in RSV-NET and 2,984 in NVSN) were identified among children aged <5 years; these included 11,681 during 2018–20 and 6,708 during 2024–25. Median patient age was 6.7 months and 14.7 months in RSV-NET (p<0.001) and 6.3 months and 12.7 months in NVSN (p<0.04),
Our Trust Standards: Medical Advisory Committee


.jpg)


.jpg)