Needle-Free Vaccinations Preferred by Nurses and Patients

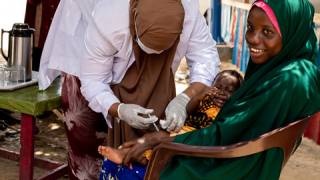
The first instance of determining the acceptability and feasibility of incorporating the Tropis needle-free jet injector for intradermal administration of fractional dose poliovirus vaccine (fIPV) produced very positive results.
PharmaJet® today announced the publication of a study demonstrating the benefits of the PharmaJet Tropis needle-free system compared with needle and syringe (N/S) for intradermal administration of fIPV.
Tropis was preferred over N/S by significant margins by both parents (98%) and nurses (98.5%) in Cuba.
Survey respondents cited ease of vaccination, diminished crying, and increased comfort as the benefits of Tropis. Of note, seroprevalence did not differ significantly between Tropis and N/S.
The results indicated significantly fewer adverse events with Tropis (6%) compared to N/S (13%) (p = 0.028).
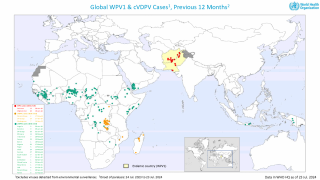
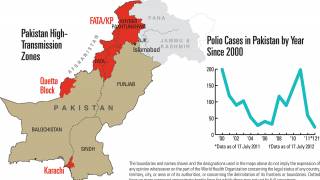
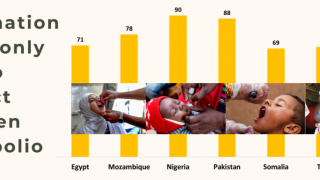
Paul LaBarre, Vice President of Global Business Development, PharmaJet, commented in a press release on May 1, 2025, “The strong performance of Tropis ID delivery is consistent with results recently seen in WHO-sponsored polio campaigns in Pakistan, Nigeria, and Somalia where Tropis has been used to administer polio vaccinations to nearly 12 million children.”
The study, entitled "Tropis needle-free injector for fractional-dose IPV administration: A pilot study for integration into routine immunization services in Cuba," is published in the journal Vaccine on April 11, 2025.
This was a community-based feasibility survey and poliovirus antibody seroprevalence assay conducted in Camagüey and Ciego de Ávila provinces, Cuba, in 2019–2020 as part of a research collaboration between the Instituto Pedro Kourí in Havana, Cuba, and the World Health Organization.
Our Trust Standards: Medical Advisory Committee

.png)
.jpg)







.png)
.png)

