U.S. Government Adopts Chikungunya Vaccine Recommendation

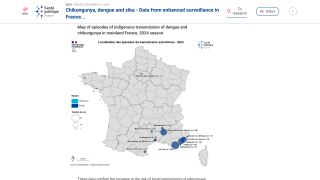
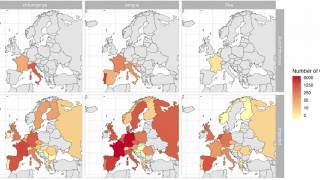
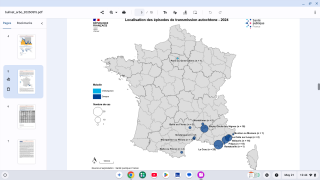
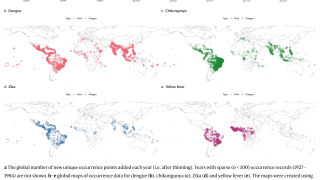
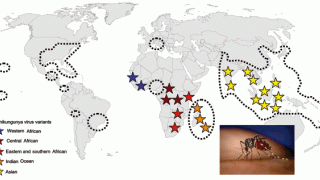
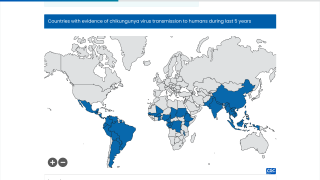
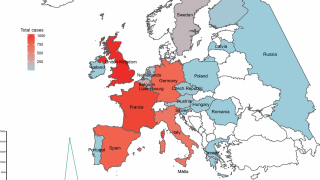
With the various countries reporting chikungunya virus outbreaks in 2025, the U.S. government has announced reassuring news regarding disease prevention.
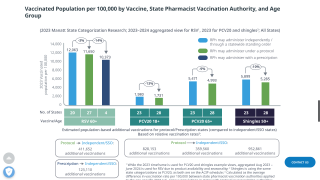
In April, the independent vaccine committee issued recommendations for using chikungunya vaccines. Effective May 13, 2025, the Health and Human Services (HHS) Secretary adopted the recommendations, which are now the official recommendations of the U.S. CDC.
The Advisory Committee on Immunization Practices (ACIP) recommends the live attenuated chikungunya vaccine for persons aged ≥18 years traveling to a country or territory with a chikungunya outbreak. In addition, the live attenuated chikungunya vaccine may be considered for persons aged ≥18 years traveling or residing in a country or territory without an outbreak but with elevated risk for U.S. travelers if planning travel for an extended time, such as 6 months or more.
Additionally, the ACIP recommends the virus-like particle chikungunya vaccine for persons aged ≥12 years traveling to a country or territory where there is a chikungunya outbreak. In addition, the virus-like particle chikungunya vaccine may be considered for persons aged ≥12 years traveling or taking up residence in a country or territory without an outbreak but with elevated risk for U.S. travelers if planning travel for an extended period, such as six months or more.
As of May 15, 2025, travel vaccination appointments are commercially available at clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee


-2.png)
-2.jpg)



.jpg)