FDA's Vaccine Committee Meets Next Week

As the United States prepares for the next wave of COVID-19 disease, the government is taking steps to clarify what changes to the formula of preventive vaccines are needed.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) recently confirmed it will meet on May 22, 2025, in open session to discuss and recommend selecting the 2025-2026 formula for COVID-19 vaccines for use in the U.S.
This VRBPAC meeting will be held virtually, from 8:30 a.m. to 4:30 p.m. ET, and is open to the public to attend digitally.
The U.S. Food and Drug Administration (FDA) intends to make background material available to the public no later than two business days before this meeting.
Recently, the new FDA Commissioner Marty Makary stated that Vinay Prasad, the director overseeing vaccines, intends to clarify the FDA’s expectations for vaccine development and approval.
For example, the FDA has asked Novavax Inc. to conduct a new randomized controlled trial for its non-mRNA COVID-19 vaccine.
Earlier this week, the World Health Organization (WHO) Technical Advisory Group on COVID-19 Vaccine Composition advised manufacturers that monovalent JN.1 or KP.2 vaccines remain appropriate vaccine antigens; monovalent LP.8.1 is a suitable alternative vaccine antigen.
The WHO wrote, 'Overall, ' the currently approved monovalent JN.1 or KP.2 vaccines continue to elicit broadly cross-reactive immune responses to circulating JN.1-derived variants.'
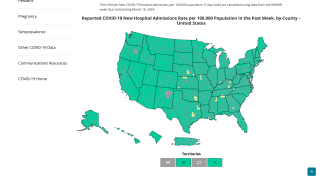
As of May 16, 2025, COVID-19 vaccines are available at various clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee








.jpg)

.jpg)



.jpg)