Breaking News
Protein-based COVID-19 Vaccine Approved
May 18, 2025 • 4:14 am CDT
.jpg)
by Gerd Altmann
(Vax-Before-Travel News)
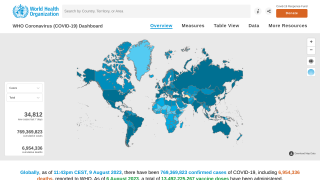
The U.S. Food and Drug Administration (FDA) announced it has approved Novavax's Nuvaxovid COVID-19 vaccine.
As of May 17, 2025, Nuvaxovid is indicated in the U.S. to prevent COVID-19 in those aged 65 years and older and for those aged 12-64 years who have one or more underlying conditions that put them at a high risk of developing COVID-related severe outcomes.
On December 17, 2021, the World Health Organization granted an Emergency Use Listing for Novavax's vaccine, and it has been available under an emergency use authorization in the United States.
As of May 18, 2025, Novavax's vaccine is the only non-mRNA COVID-19 vaccine available at clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee








.jpg)

.jpg)



.jpg)