Researchers believe the yellow fever virus has existed for thousands of years, originating in Africa and spreading to the Region of the Americas in the 17th century.
As of May 14, 2025, the Pan American Health Organization (PAHO) says yellow fever has become endemic in 13 countries in the Americas.
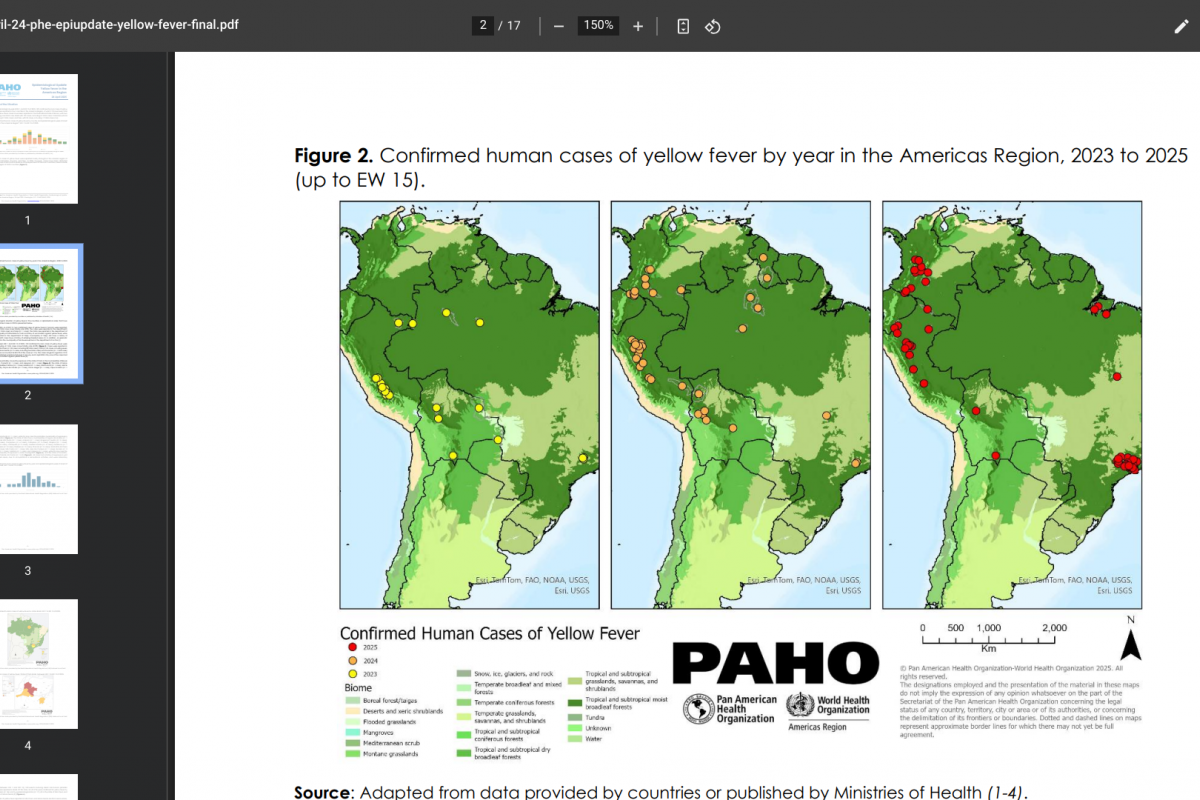
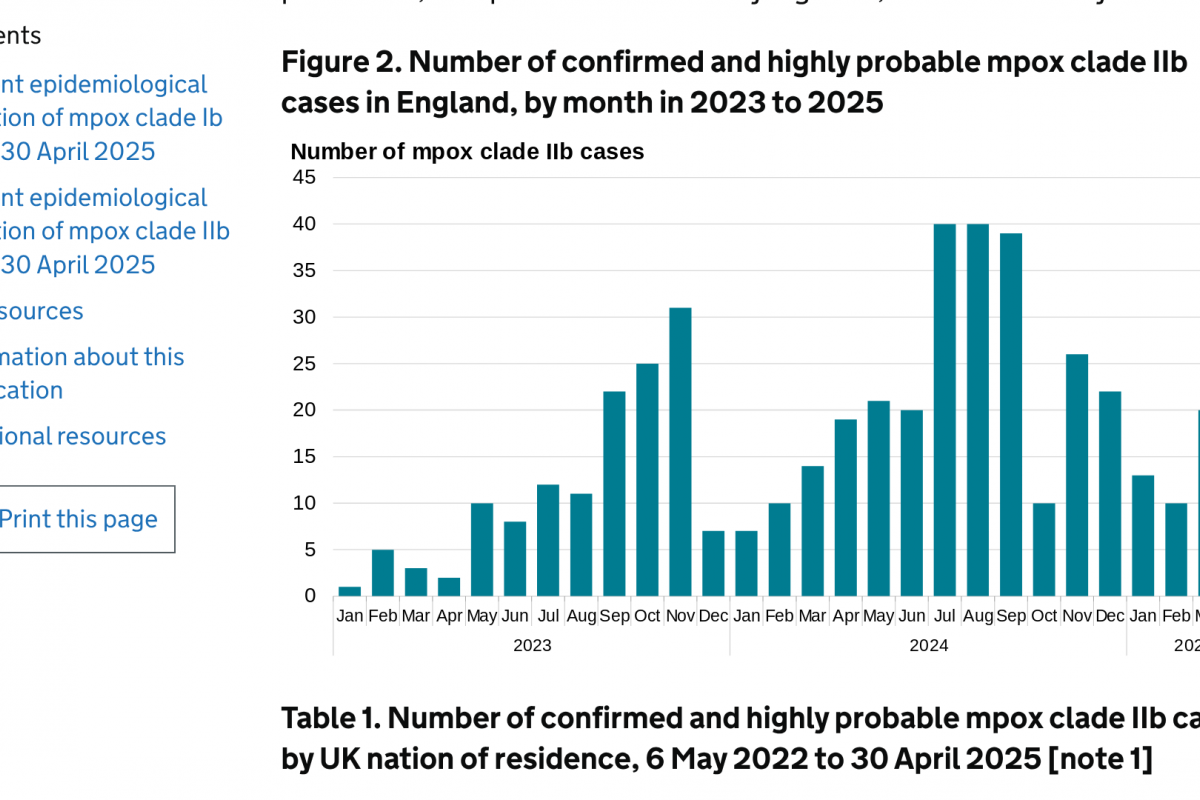
The PAHO reported 189 confirmed human cases of yellow fever (YF) in four countries in the Americas Region, of which 74 have been fatal.
The breakdown of reported YF cases is as follows: The Plurinational State of Bolivia, with two cases, including one fatal case; Brazil with 102 cases, including 41 fatal cases; Colombia with 53 cases, including 21 fatal cases; and Peru with 32 cases, including 11 fatal cases.
In 2025, YF cases were detected mainly in the state of São Paulo in Brazil and the department of Tolima in Colombia, areas outside the Amazon region of both countries.
The PAHO/WHO encourages Member States to continue surveillance and vaccination efforts in YF-endemic areas.
'It is essential that countries achieve vaccination coverage of at least 95% in populations in at-risk areas,' writes the PAHO.
And that health authorities ensure that they have a strategic reserve inventory that allows them to maintain routine YF vaccination and, at the same time, respond effectively to possible outbreaks.
In the United States, YF vaccination appointments are offered at travel clinics and pharmacies in 2025.